Trials Test Survivors’ Blood as COVID-19 Treatment
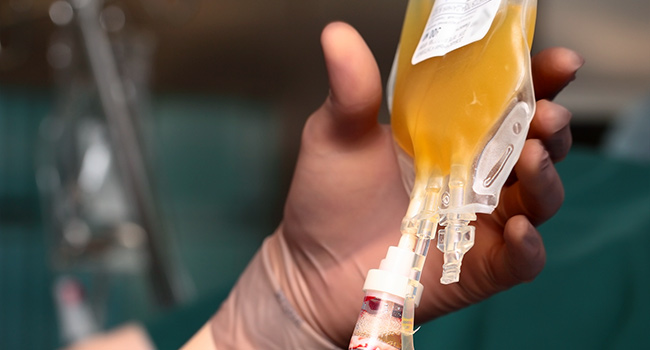
“Blood plasma is the liquid part of the blood,” explained Dr. Arturo Casadevall, chair of Hopkins’ department of molecular microbiology and immunology and lead researcher on the two Hopkins studies.
Characterized by its yellowish cast, plasma contains both red and white blood cells, as well as the colorless platelets the body deploys to clot and stem bleeding from wounds or cuts.
“And that’s [also] where the antibodies are,” Casadevall noted. “The antibodies are floating in the liquid.”
With plasma therapy, he explained, “you’re taking the antibodies that somebody else made when they recovered and you’re transferring them to a new person. So, the new person gets them already made, and can use them right away.”
Casadevall said the practice of extracting convalescent plasma from survivors of a disease and giving it to patients either as a preventive measure or as a treatment has a “long history” in medicine.
“It’s been used for 120 years,” he said. “The first Nobel Prize was given for it. This is how [far] back it goes.”
Grudzen also noted convalescent plasma’s long history in medicine, but added that, “It’s not without potential harms.”
Convalescent plasma not without dangers
The two main ones are the patient catching an infectious disease, though blood screening helps to lessen that possibility, or a patient’s immune system reacting badly to the injected plasma of another person. Grudzen noted that, “especially in older people, it’s a little more risky.”
And Casadevall cautioned that plasma therapy still needs to be tested on COVID-19 to see if it’ll work against this specific virus, “simply because it’s a new organism.”
To that end, Hopkins is set to start two COVID-19 plasma investigations simultaneously, with funding coming from Bloomberg Philanthropies, the state of Maryland, and Hopkins.
“The first trial is focused on trying to use plasma on high-risk populations,” he noted. That investigation will include health care workers on the frontlines of the pandemic and nursing home residents, with a minimum of 150 participants.
“The second trial is focused on people who are sick at home,” Casadevall said. “We know most people recover. Most people today who are sick are sitting at home hoping to get better. But we also know that some fraction of them will get worse,” he added.
Source link
